
Types of Batteries: Key Takeaways
- The most common battery types used in electronics include lithium-ion (Li-ion), nickel-metal hydride (NiMH), alkaline, lead-acid, and lithium primary cells
- Battery types vary by chemistry, rechargeability, and performance, with li-ion, NiMH, alkaline, and lead-acid accounting for over 95% of global battery usage in electronics
- Primary batteries (non-rechargeable) offer up to 10 years of shelf life, while secondary batteries (rechargeable) can deliver 300–5,000+ cycles, depending on chemistry
- Lithium-based chemistries dominate high-performance electronics, offering high energy density and compact form factors for mobile, medical, and EV applications
- Battery selection should be driven by application needs, including load profile, environment, power demand, and maintenance limitations
- Partnering with sourcing experts like AGS Devices ensures battery availability, spec compliance, and lifecycle support across industrial, consumer, and medical sectors
Over 70% of product failures in portable electronics can be traced back to power system issues, and battery choice is often the root cause.
That’s because different types of batteries behave differently under load, temperature, and time.
Behind every reliable device lies one crucial decision: choosing the right battery chemistry and format.
Today, we’ll break down:
- The most common battery types used in electronics
- Primary vs. secondary cells and how they differ
- Key battery chemistries like Li-ion, NiMH, and alkaline
- How to match battery performance to your application
- Specialty and small-format battery types to consider
Different Types of Batteries Used in Electronics
Battery types in electronics fall into two broad categories: primary (non-rechargeable) and secondary (rechargeable).
Here’s a breakdown of the most common types:
| Battery Type | Chemistry | Rechargeable? | Typical Use |
| Alkaline | Zinc-manganese dioxide | No | Remote controls, flashlights, toys |
| Lithium primary | Lithium-manganese dioxide | No | Medical devices, memory backup |
| Nickel-cadmium (NiCd) | Nickel-cadmium | Yes | Power tools, two-way radios |
| Nickel-metal hydride | Nickel-metal hydride | Yes | Consumer electronics, hybrid vehicles |
| Lithium-ion (Li-ion) | Lithium cobalt oxide | Yes | Smartphones, laptops, electric vehicles |
| Lithium iron phosphate | LiFePO₄ | Yes | Power tools, solar storage, EVs |
| Lead-acid | Lead dioxide | Yes | UPS systems, automotive, industrial |
Each battery type offers trade-offs in voltage, capacity, cycle life, and environmental resilience.
For instance:
- Alkaline is low-cost and reliable for intermittent use
- Li-ion offers high energy density but requires tight charge control
- NiMH balances capacity and affordability but has memory effect risks

Primary vs. Secondary Batteries: Pros and Cons
Batteries are classified as primary or secondary based on whether they can be recharged.
Primary Batteries (Non-Rechargeable)
These are single-use cells designed for long shelf life and consistent voltage output. They excel in low drain, infrequently used devices.
Common chemistries: Alkaline, lithium-manganese dioxide, zinc-carbon
Advantages:
- High energy density
- Long shelf life (up to 10 years)
- Lower self-discharge rates
- Ideal for emergency and backup systems
Limitations:
- Cannot be recharged
- Higher lifetime cost in high-use devices
- Limited current output compared to rechargeables
Use cases: Remote controls, smoke detectors, military gear, medical implants
Secondary Batteries (Rechargeable)
These batteries are designed for multiple charge-discharge cycles and are the backbone of portable and mobile electronics.
Common chemistries: Lithium-ion, nickel-metal hydride, lead-acid
Advantages:
- Rechargeable 300–1,000+ times
- Lower long-term cost
- Higher discharge rates for demanding loads
Limitations:
- Shorter shelf life
- Requires charge management circuits
- Typically higher upfront cost
Use cases: Laptops, electric vehicles, power tools, grid storage
Battery Chemistries Explained: Li-ion, NiMH, Alkaline & More
Not all batteries are created equal. Their internal chemistry directly impacts how they perform in real-world devices.
Here’s a closer look at the most important types, with specific use cases engineers rely on.
Lithium-Ion (Li-ion): The High-Density Powerhouse
Found in nearly every mobile and portable device, Li-ion cells offer superior energy density and compact packaging.
Why engineers choose it:
- Ideal for space-constrained designs
- Supports high-drain and fast-charging applications
- Proven performance in harsh usage cycles
You’ll find it in smartphones, laptops, drones, and electric vehicles (EVs).
One caveat: These cells are heat-sensitive and require proper thermal management, which requires battery management systems (BMS) for safety
Nickel-Metal Hydride (NiMH): A Rechargeable Workhorse
NiMH is often used as a drop-in replacement for alkaline batteries in AA and AAA sizes, with the added benefit of rechargeability.
Engineers value this chemistry for its:
- Reliable mid-drain devices
- Standardized consumer formats availability
- Environmentally safe compared to NiCd
You’ll find it in digital cameras, handheld scanners, and hybrid cars
Watch out for: Self-discharge and some susceptibility to memory effect
Alkaline: Low-Cost, High-Shelf-Life Primary Cells
One of the most widely used chemistries in the world, alkaline batteries offer stability and affordability for low-drain or intermittent-use devices.
Quite useful because they have:
- Low upfront cost
- Shelf lives of up to a decade
- Great for infrequently used equipment
You’ll find it in remote controls, thermometers, toys, and wall clocks
Watch out for: They are non-rechargeable and unsuitable for power-hungry or continuous-use systems
Lithium Iron Phosphate (LiFePO₄): Long-Life and Safety First
This Li-ion alternative is rising in popularity for energy storage and high-cycle environments.
You might choose this because of:
- Extremely stable thermal profile
- Exceptional cycle life (2,000+)
- Good performance under load
You’ll find it in home solar systems, power tools, and light EVs
Watch out for: Lower voltage and energy density compared to standard Li-ion

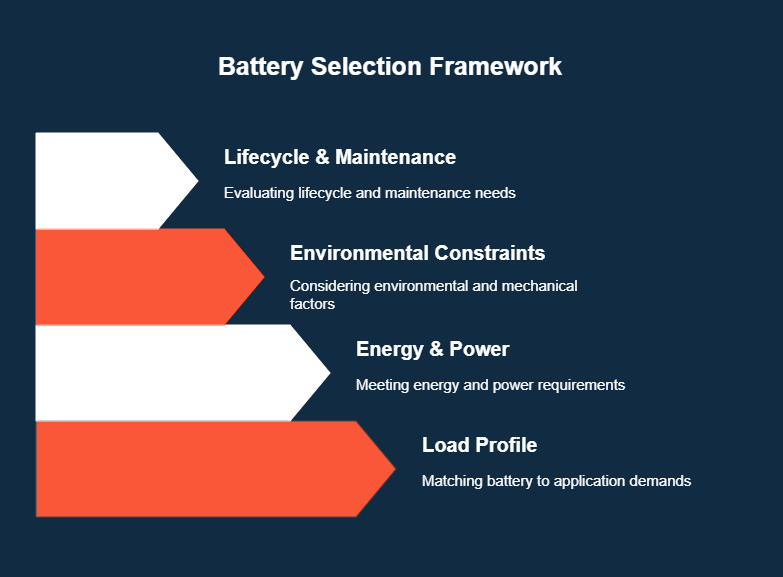
How To Select the Right Battery for Your Application: 4 Steps
Choosing the wrong battery can tank your product’s performance, inflate costs, or cause reliability issues in the field.
To avoid this, engineers need to match battery specs with use-case demands, environmental conditions, and system constraints.
Here’s a performance-first framework for selecting the right battery:
1. Define the Load Profile
Start with how the battery will be used.
Key questions:
- Is the device continuous-use or standby?
- What’s the average vs. peak current draw?
- Does the load fluctuate or stay steady?
If you need:
- Long, low-current operation → Alkaline or lithium primary
- High-drain, short bursts → Li-ion or NiMH
2. Determine Energy and Power Requirements
Check voltage, capacity (mAh or Ah), and discharge rate.
Engineering tips:
- For voltage-sensitive circuits, select batteries with tight voltage tolerance
- C-rate tells you how fast a battery can safely discharge
If your design demands:
- High energy density → Li-ion
- Power surges (e.g., motors) → Lead-acid or LiFePO₄
3. Account for Environmental and Mechanical Constraints
Where and how your product operates will shape battery choice.
Considerations:
- Temperature range
- Vibration/shock exposure
- Humidity and ingress protection
If operating in:
- High-heat or rugged environments → LiFePO₄ or sealed lead-acid
- Compact spaces → Button cells, prismatic Li-ion
4. Evaluate Lifecycle and Maintenance Needs
Some applications require zero maintenance, while others demand swap/recharge cycles.
Think about:
- Expected runtime per charge
- Recharge time allowed
- Maintenance or access restrictions
Choose:
- Rechargeables (Li-ion, NiMH) for long-term use
- Primaries for sealed or hard-to-access products
Reliable Battery Component Sourcing Starts With AGS Devices
At AGS Devices, we understand that battery performance is only as strong as the electronic components and connections behind it.
From medical wearables to industrial robotics and automotive systems, every power system demands precision, quality, and trusted sourcing.
Whether you’re designing for longevity, scaling production, or adapting to supply chain challenges, we support your entire workflow.
We provide:
From BOM optimization to sourcing specialty cells and components, AGS Devices ensures your power systems perform reliably, efficiently, and on time.
Types of Batteries: FAQs
What’s the difference between a dry-cell battery and a wet-cell battery?
Dry-cell batteries use a paste electrolyte, making them spill-proof and suitable for portable electronics. Wet-cell batteries contain liquid electrolyte and are typically used in automotive or industrial settings where weight and orientation aren’t critical.
What are the 3 main types of batteries used in electronics?
The three most common types are:
- Lithium-ion (Li-ion): High energy density, rechargeable
- Nickel-Metal Hydride (NiMH): Rechargeable, safer than NiCd
- Alkaline: Primary (non-rechargeable), cost-effective for low-drain use
How do battery cell types impact circuit design?
Battery type influences voltage thresholds, current delivery, charge management, and thermal design. Engineers must ensure battery specs align with regulator and load requirements to avoid undervoltage lockout or overheating.
What is a “button cell” battery, and where is it used?
Button cells are small, coin-shaped primary batteries, often based on lithium or silver oxide chemistry. They’re used in watches, hearing aids, key fobs, and sensors, where space is limited but long life is needed.
Can you mix battery chemistries in the same device?
No. Mixing different chemistries (e.g., NiMH with alkaline) can lead to uneven discharge, leakage, or device failure. Always use identical batteries from the same batch when designing or replacing cells.
